Covalent Bonds
-electrons are shared between non-metals
-to draw Lewis Dot Diagram:
1. Add the valence e- in all atoms
2. Identify which atom can form the most number of bonds. This will be the central atom
3. Bonds between two atoms are represented by a e-. This represents 2e-
4. Any e- not creating bonds are placed in pairs around the remaining atoms
5. All valences levels must be filled, all electrons must be used
Double and Triple Bonds
- some compounds from more than one bond between two elements
http://www.youtube.com/watch?v=tOpke6cpqWY&feature=pyv&ad=4006919948&kw=covalent%20bonds
http://www.youtube.com/watch?v=mAjrnZ-znkY
Electronegativity
- atoms affinity for electrons
- electronegativity increase from left to right and from bottom to top
Atoms and Ions
Mar 22, 2010
at
8:25 PM
| Posted by
blk A chemists
-atoms are electically neutral
-# of protons = # of electrons
-ions have different # of protons and electrons
-ions cane either be positive (lost e) or negative (extra e)
-cation = positive ion
-anion = negative ion
EX. Determine how many electrons each of the ions have. What type of ion are they(cation/anion)?
-Ca2+ lost 2e, cation, 18e
-Ag+ lost 1e, cation, 46e
EX. Determine how many protons,neutrons,electrons the following substances have.
-76As3- p=33 n=43 e=36
-201Au+ p=79 n=122 e=78
Bohr Diagrams for Ions
-energy level bohr diagram
8e <--- Ca+ 8e 2e 40Ca 20 Chemical Bonds
-a bond is an electrostatic attraction between particles
-bonds occur as elements try to achieve noble gas electron configuration
---> noble gases (usually) dont form compounds/bonds
---> in noble gasese the outermost energy level have stable octets
-metals lose electrons (oxidize)
-non-metals gain electrons (reduced)
Lewis Dot structure
-atoms can be repersented by dot diagrams
---> dots represent electrons
---> only valence electrons shown
-write the atomic symbol for the atom
---> this represents the nucleus and filled inner electrons levels
-one dodt is used to represent outer energy level elctrons
---> one e is placed in each orbital before any pairing occurs
---> beginning with the 5th e, pairing can occur up to max. of 8e
EX.
Ionic Bond
-electrons are transtered from metal to non-metal
---> no dots are shown on metal
-"charged" species is written in brackets
EX.
-# of protons = # of electrons
-ions have different # of protons and electrons
-ions cane either be positive (lost e) or negative (extra e)
-cation = positive ion
-anion = negative ion
EX. Determine how many electrons each of the ions have. What type of ion are they(cation/anion)?
-Ca2+ lost 2e, cation, 18e
-Ag+ lost 1e, cation, 46e
EX. Determine how many protons,neutrons,electrons the following substances have.
-76As3- p=33 n=43 e=36
-201Au+ p=79 n=122 e=78
Bohr Diagrams for Ions
-energy level bohr diagram
8e <--- Ca+ 8e 2e 40Ca 20 Chemical Bonds
-a bond is an electrostatic attraction between particles
-bonds occur as elements try to achieve noble gas electron configuration
---> noble gases (usually) dont form compounds/bonds
---> in noble gasese the outermost energy level have stable octets
-metals lose electrons (oxidize)
-non-metals gain electrons (reduced)
Lewis Dot structure
-atoms can be repersented by dot diagrams
---> dots represent electrons
---> only valence electrons shown
-write the atomic symbol for the atom
---> this represents the nucleus and filled inner electrons levels
-one dodt is used to represent outer energy level elctrons
---> one e is placed in each orbital before any pairing occurs
---> beginning with the 5th e, pairing can occur up to max. of 8e
EX.

Ionic Bond
-electrons are transtered from metal to non-metal
---> no dots are shown on metal
-"charged" species is written in brackets
EX.

|
0
comments
|
![]()
Trends in Chemical Properties on the Periodic Table
Mar 20, 2010
at
10:01 PM
| Posted by
lacheeeks
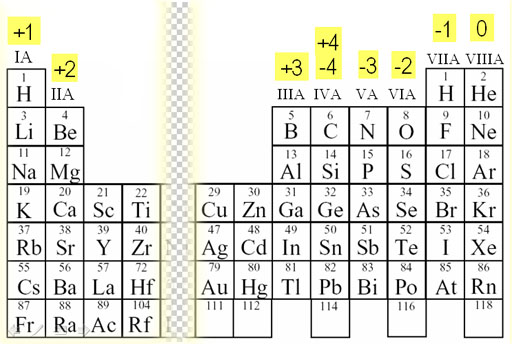 Ion Charge: the electrical charge that forms on an atom when it gains or loses electrons
Ion Charge: the electrical charge that forms on an atom when it gains or loses electrons- elements on the left side of the Periodic Table tend to form positive ions (cations)
- elements on the right side of the Periodic Table tend to form negative ions (anions)
- elements are arranged in columns or families by their similar ion charge(s)
- from the left side, elements begin with a positive charge that increases down the right of the period until it reaches a non-metal
- from the right side, elements begin with a negative charge that increases negatively to the left of the period until it reaches a metal
- the transition metals which have been positioned in the center (indicated on the chart by a solid checkered line) because most of them have more than one charge
Chemical Reactivity: the rate at which a chemical substance tends to undergo a chemical reaction time
- for metals, chemical reactivity increases as you move towards the left and down
- for non-metals, chemical reactivity increases as you move towards the right and up
ex. Potassium is more reactive than Beryllium because it is farther to the left and farther down. (Potassium also has much more electrons and is only one electron away from reaching a full valence shell.)
Ionization Energy: the energy needed to remove electrons from an atom; also referred to as ionization potential
- an atom with layers of inner electrons has stronger ionization energy because the inner electrons protect the outer electrons from the force of attraction the protons give off
ex.Chlorine has stronger ionization energy than Lithium

|
1 comments
|
![]()
Chemical Families
Mar 17, 2010
at
7:42 PM
| Posted by
lacheeeks
Vertical columns are the groups or chemical families: Alkali Metals, Alkaline Earth Metals, Transition Metals, Halogens and Noble Gases
- Hydrogen is its own group
- elements in the same chemical family have similar physical and chemical properties.

Alkali Metals
- in group 1
- highly reactive and reactivity increases as you go down
- have only one electron in their outer shell and are lose that electron in ionic bonding with other elements
- react with non-metal
- usually have lower densities than other metals
- malleable, ductile, good conductors of heat and electricity
- have low melting points, below 200 °C
- soft and can be cut with a knife
Alkaline Earth Metals
- 2nd group
- have two electrons in their outer shell
- have low electronegativities
- less reactive than Alkali Metals but they will burn in air if heated. T
- react with water
- shiny
Transition Metals
- are the 38 elements in groups 3-12 of the periodic table
- are very hard
- have high melting and boiling points
- low ionization energies
- high electrical conductivity
- are malleable, they can be shaped and bent.
The Halogens
- in group 17 of the periodic table
- are highly reactive non-metals with strong and unpleasant odours
- will burn flesh and do not react well with water
- fluorine and chlorine are gases at room temperature, bromine is a liquid and iodine and astatine are solids.
Noble Gases
- group 18
- most stable and unreactive elements
- colourless, odourless gases at room temperature
- have high ionization energies and low boiling points
- Hydrogen is its own group
- elements in the same chemical family have similar physical and chemical properties.

Alkali Metals
- in group 1
- highly reactive and reactivity increases as you go down
- have only one electron in their outer shell and are lose that electron in ionic bonding with other elements
- react with non-metal
- usually have lower densities than other metals
- malleable, ductile, good conductors of heat and electricity
- have low melting points, below 200 °C
- soft and can be cut with a knife
Alkaline Earth Metals
- 2nd group
- have two electrons in their outer shell
- have low electronegativities
- less reactive than Alkali Metals but they will burn in air if heated. T
- react with water
- shiny
Transition Metals
- are the 38 elements in groups 3-12 of the periodic table
- are very hard
- have high melting and boiling points
- low ionization energies
- high electrical conductivity
- are malleable, they can be shaped and bent.
The Halogens
- in group 17 of the periodic table
- are highly reactive non-metals with strong and unpleasant odours
- will burn flesh and do not react well with water
- fluorine and chlorine are gases at room temperature, bromine is a liquid and iodine and astatine are solids.
Noble Gases
- group 18
- most stable and unreactive elements
- colourless, odourless gases at room temperature
- have high ionization energies and low boiling points
|
0
comments
|
![]()
Mendeleev's Periodic Table
Mar 15, 2010
at
7:53 PM
| Posted by
lacheeeks
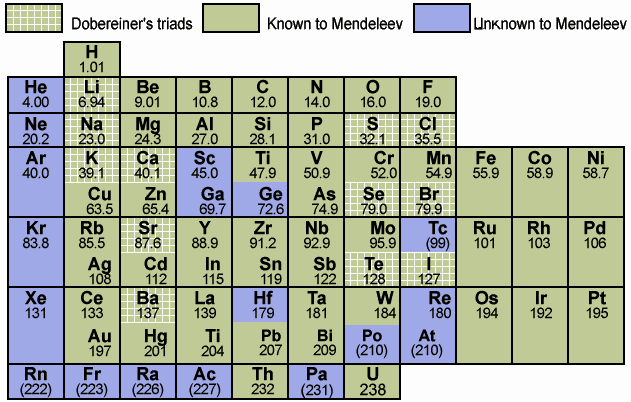
- Dmitri Mendeleev started organizing every known element and is credited with creating the first real periodic table of elements
- he sawa pattern and left spaces in the table for elements that were undiscovered
- there are 118 elements on the Periodic Table
- 7 periods and 18 columns
- metals on the left and non-metals are on the right
- elements with similar properties are in the same vertical column
- Atomic Number: Number of protons of the nucleus of each atom of an element
- Atomic Mass: Mass of an average atom of an element (tends to increase along with atomic number)
- Ion charge: Electric charge that forms on an atom when it gains or loses electrons
- he sawa pattern and left spaces in the table for elements that were undiscovered
- there are 118 elements on the Periodic Table
- 7 periods and 18 columns
- metals on the left and non-metals are on the right
- elements with similar properties are in the same vertical column
- Atomic Number: Number of protons of the nucleus of each atom of an element
- Atomic Mass: Mass of an average atom of an element (tends to increase along with atomic number)
- Ion charge: Electric charge that forms on an atom when it gains or loses electrons

|
0
comments
|
![]()
Where's Mr. Doktor?
Mar 11, 2010
at
11:44 AM
| Posted by
lacheeeks
Mr. Doktor wasn't in class today and we had a sub. Instead of reviewing homework, we were assigned our group chemistry projects and started working on them.
Just a quick recap:
This is how two isotopes of hydrogen would look like. In essence, most of their properties are retained except for certain structural details.

Just a quick recap:
This is how two isotopes of hydrogen would look like. In essence, most of their properties are retained except for certain structural details.

|
0
comments
|
![]()
Emission Spectra
Mar 8, 2010
at
8:48 PM
| Posted by
blk A chemists
-each element gives of a specific colour of light
-these are known as emission spectra, unique to each element
-If electrons absorb energy they can bumped to a higer level
-when the fall to a lower level, they release energy as light
Atomic Structure:
-atoms are made up parts called subatomic particles:
1)protons- positive, mass 1, located in nucleus
2)neutrons- neutral, mass 1, located in nucleus
3)electrons- negative, mass 1/1837, located outside

Atomic Number:
-atomic number is the number of protons

Isotopes:
-the number of protons dertermine they type of element
-changing number of neutrons chages isotopes of the element
-all isotopes have the same chemical properites

Mass Number:
-mass number is the total number of protons and neutrons
-give symbol A
-different isotopes have different masses
-mass number = atomic number + number of neutrons
-A = Z+N
-these are known as emission spectra, unique to each element
-If electrons absorb energy they can bumped to a higer level
-when the fall to a lower level, they release energy as light
Atomic Structure:
-atoms are made up parts called subatomic particles:
1)protons- positive, mass 1, located in nucleus
2)neutrons- neutral, mass 1, located in nucleus
3)electrons- negative, mass 1/1837, located outside

Atomic Number:
-atomic number is the number of protons

Isotopes:
-the number of protons dertermine they type of element
-changing number of neutrons chages isotopes of the element
-all isotopes have the same chemical properites

Mass Number:
-mass number is the total number of protons and neutrons
-give symbol A
-different isotopes have different masses
-mass number = atomic number + number of neutrons
-A = Z+N
|
0
comments
|
![]()
Bohr Model
Mar 4, 2010
at
8:46 AM
| Posted by
blk A chemists
- atoms are electrically neutral
-two different models can be used to describe electron configuration
i) Energy Level Model
ii) Bohr Model
-Electrons occupy or orbitals
i) 2 e in the first orbital
ii) 8 e in the second orbital
iii) 8 e in the third orbital
- these are called octet
Hybridized Orbitals
- the first of the Bohr Levels is the 1s-orbital and it holds 2e-
- the second level contains the 2s, 2px, 2py, 2pz orbitals. They combine (hybridize) to from one 2sp3 orbital
-two different models can be used to describe electron configuration
i) Energy Level Model
ii) Bohr Model
-Electrons occupy or orbitals
i) 2 e in the first orbital
ii) 8 e in the second orbital
iii) 8 e in the third orbital
- these are called octet
Hybridized Orbitals
- the first of the Bohr Levels is the 1s-orbital and it holds 2e-
- the second level contains the 2s, 2px, 2py, 2pz orbitals. They combine (hybridize) to from one 2sp3 orbital
|
0
comments
|
![]()
Early Atomic Theory
Mar 1, 2010
at
9:36 PM
| Posted by
lacheeeks
 In today's class, Mr. Doktor discussed atomic theory. There were seven significant people who's ideas contributed to the development of atomic theory: the Greeks, Lavoisier (late 1700s), Proust(1799), Dalton (early 1800s), J.J. Thomson (1850s), Rutherford (1905), and Bohr (1920s).
In today's class, Mr. Doktor discussed atomic theory. There were seven significant people who's ideas contributed to the development of atomic theory: the Greeks, Lavoisier (late 1700s), Proust(1799), Dalton (early 1800s), J.J. Thomson (1850s), Rutherford (1905), and Bohr (1920s).Some important points..
The Greeks:
- in 3000 BC, Democritus said that atoms were indivisible particles
- first mention of atoms
- only a conceptual model
- was the accepted view for over 2000 years
Lavoisier:
- Law of conservation of mass
- Law of definite proportions
*wasn't a true atomic theory because it did not discuss what atoms were or how they were arranged
Proust:
- if a compound is broken down into its constituents, the products exist in the same ratio as in the compounded form
- experimentally proved Lavoisier's Laws
 Dalton:
Dalton:- atoms are solid, indestructible spheres (like billiard balls)
- provides for different elements
J. J. Thompson:
- used the raisin bun model
-solid, positive spheres with negative particles embedded in them
- first atomic theory to have positive (protons) and negative (electron) charges
- introduced the idea of nucleus
Rutherford:
- showed that atoms have a positive, dense center with electrons outside it
- resulted in a planetary model
- explains why electrons spin around nucleus
- suggests atoms are mostly empty space
- should be unstable (electrons and protons should attract and destroy the atom)
Bohr:- electrons must only exist in specific orbitals around nucleus
- explains how valence electrons are involved in bonding
- explains the difference between ionic and covalent bonding
- resolves the problem of atomic instability
- includes the neutron (discovered in 1932)
- explains atomic emission spectra
Review Questions:
1. Who first mentioned atoms? Democritus
2. Who introduced the idea of nucleus? J. J. Thomson
3. Who stated that atoms were indestructible? Dalton
4. Who explained bonding and 'levels' around the nucleus? Bohr
5. Who explained why electrons spin around the nucleus? Rutherford

|
0
comments
|
![]()
