In today's class, we created a smell in our Esterfication lab. My group chose
Here's a quick review if you do not remember how Esterfication works:
Amines and Amides
May 20, 2010
at
8:12 PM
| Posted by
lacheeeks

Amines:
end in -amine after the alkyl prefix
have a double bonded oxygen to the carbon chain and NH2 connected to the carbon chain
Name the following:

Answers:
1. ethanamide
2. methanamide

Amides:
end in -amide with the carbon prefix
have possibilities of primary (one carbon chain), secondary (two carbon chains), or tertiary (three carbon chains) chains connected to nitrogen
Name the following:


Answers:
1. dimethylamine
2. methylamine
|
0
comments
|
![]()
Aldehydes, Carboxylic Acids, Esters & Esterfication
at
12:01 PM
| Posted by
lacheeeks
ALDEHYDES
Naming: change the -e ending to -al

Ex. Draw Methanal (formaldehyde)

Ex. Benzaldehyde

CARBOXYLIC ACIDS
Naming: change the ending to -oic acid

Examples:

ESTERS
Naming: the primary chain has the -yl ending and the secondary chain takes the -oate ending
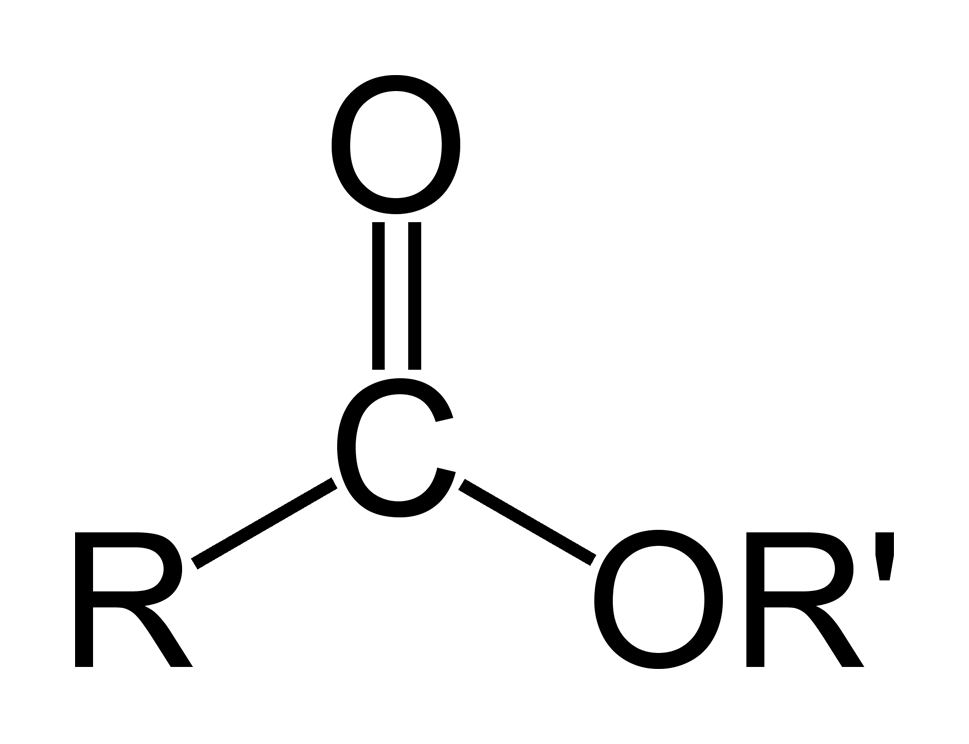
Examples:

ESTERFICATION
Always has H2O

Check out this website for some help!
http://www.ausetute.com.au/esters.html
Naming: change the -e ending to -al

Ex. Draw Methanal (formaldehyde)

Ex. Benzaldehyde

CARBOXYLIC ACIDS
Naming: change the ending to -oic acid

Examples:
ESTERS
Naming: the primary chain has the -yl ending and the secondary chain takes the -oate ending

Examples:

ESTERFICATION
Always has H2O

Check out this website for some help!
http://www.ausetute.com.au/esters.html
|
0
comments
|
![]()
Functional Groups
May 18, 2010
at
9:07 PM
| Posted by
blk A chemists
Halides:
-halogen atoms replace a hydrogen: Bromo, Chloro, Floro
EX. 2,2, dibromo 3 chloro, 1,3 difloro propane

Alchohols:
-OH or hydroxyl group
-change the ending to -ol
EX. 3 floro 2 methyl 4 pentaol

Ketones:
-oxygen atom doubled bonded to carbon
-change the ending -one
EX. 2 propaone (acetone)

Ethane:
-have an O joingin the two carbon chains together
-name carbon side chain with -yl ending and add 'ether'
EX. diethyl ether

-halogen atoms replace a hydrogen: Bromo, Chloro, Floro
EX. 2,2, dibromo 3 chloro, 1,3 difloro propane

Alchohols:
-OH or hydroxyl group
-change the ending to -ol
EX. 3 floro 2 methyl 4 pentaol

Ketones:
-oxygen atom doubled bonded to carbon
-change the ending -one
EX. 2 propaone (acetone)

Ethane:
-have an O joingin the two carbon chains together
-name carbon side chain with -yl ending and add 'ether'
EX. diethyl ether

|
0
comments
|
![]()
Alkenes & Alkynes
May 4, 2010
at
4:55 PM
| Posted by
lacheeeks
ALKENES
- compounds with double bonds end in -ene
- a number in front of the parent chain tells where the double bond is
- more than 1 double bond changes the parent chain slightly
ALKYNES
- for compounds with triple bonds, use the -yne ending
- follow all the same alkene rules
Here's a quick recap:
And for some practice, check out this worksheet:
Organic Chemistry (with answers)
- compounds with double bonds end in -ene
- a number in front of the parent chain tells where the double bond is
- more than 1 double bond changes the parent chain slightly
ALKYNES
- for compounds with triple bonds, use the -yne ending
- follow all the same alkene rules
Here's a quick recap:
And for some practice, check out this worksheet:
Organic Chemistry (with answers)
|
0
comments
|
![]()