If you're still a bit confused, take a look at these helpful hints!

And here's a video of a car reverse fail in case you missed out:
Have a Merry Christmas and enjoy the break!

|
0
comments
|
![]()

|
0
comments
|
![]()
|
0
comments
|
![]()
|
0
comments
|
![]()
|
0
comments
|
![]()
|
0
comments
|
![]()
|
0
comments
|
![]()
| Element | Mass (g) | Atomic Mass | Moles |
| C | 8.4 | 12.0 g/mol | 8.4g ÷ 12.0 g/mol = 0.7 mol |
| H | 2.1 | 1.0 g/mol | 2.1g ÷ 1.0 g/mol = 2.1 mol |
| O | 5.6 | 16.0 g/mol | 5.6g ÷ 16.0 g/mol = 0.35 mol |
| Element | Mass (g) | Atomic Mass | Moles |
| Ca | 13.5 | 40.1 g/mol | 13.5g ÷ 40.1 g/mol = 0.337 mol |
| O | 10.8 | 16.0 g/mol | 10.8g ÷ 16.0 g/mol = 0.269 mol |
| H | 0.675 | 1.0 g/mol | 0.675g ÷ 1.0 g/mol = 0.675 mol |
|
0
comments
|
![]()
|
0
comments
|
![]()
|
1 comments
|
![]()

|
0
comments
|
![]()
|
0
comments
|
![]()
|
0
comments
|
![]()
|
0
comments
|
![]()

|
0
comments
|
![]()

|
1 comments
|
![]()

|
0
comments
|
![]()

|
0
comments
|
![]()
|
0
comments
|
![]()
|
0
comments
|
![]()
|
1 comments
|
![]()


|
1 comments
|
![]()


|
0
comments
|
![]()

|
0
comments
|
![]()



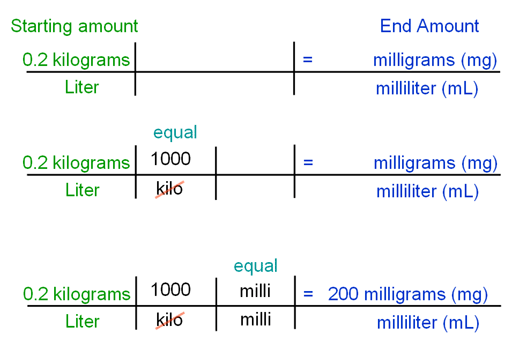
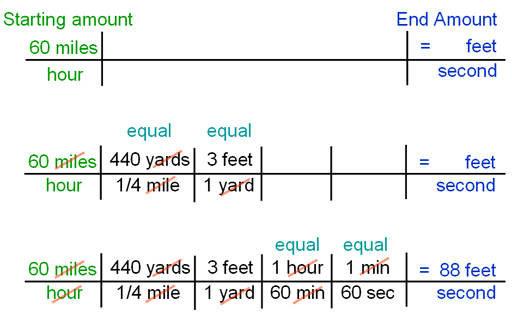

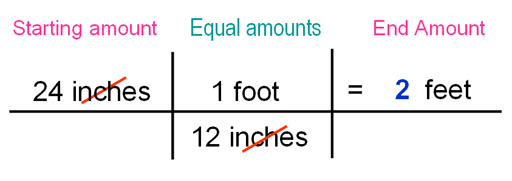
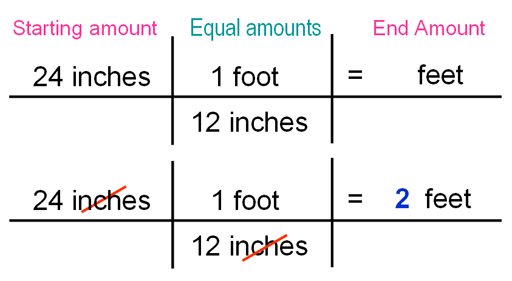
|
0
comments
|
![]()


| Trial | Volume of Water (mL) | Mass of Salt (g) |
| 1 | 10 | 0.70 |
| 2 | 20 | 1.83 |
| 3 | 30 | 2.67 |
| 4 | 40 | 3.62 |
|
0
comments
|
![]()
|
0
comments
|
![]()


|
0
comments
|
![]()